Our in-depth articles explore how our work is making a real difference to people’s lives.
Through this featured content, you’ll learn how our guidance and advice is supporting effective decision making, for the benefit of individuals and society as a whole.

Supporting evidence-based decision-making in Argentina
In this case study, we explore NICE International’s collaboration with the Argentine Ministry of Health. We've been working together to strengthen Argentina's decision-making processes in the development of evidence-based recommendations.

Ensuring the voice of primary care is heard - a committee member’s story
Dr Teik Goh, GP and NICE committee member, outlines his experience of sitting on our medical technologies advisory committee.

Becoming part of the change process - a committee member’s story
Liz Cross is an advanced nurse practitioner and NICE committee member. Liz outlines her experience of sitting on our indicator advisory committee.

Bringing evidence-based medicine into reality – a committee member’s story
Avril Tucker is a primary care antimicrobial pharmacist and NICE committee member. Avril outlines her experience of sitting on our public health advisory committee for managing common infections.

A rewarding experience – a committee member’s story
Dr Helen Parretti is a GP and NICE committee member. Dr Parretti outlines her experience of sitting on our weight management update guideline committee.

Developing clinical guidelines and quality standards in the Philippines
In this case study, we explore NICE International’s collaboration with the Philippine Department of Health. This project focuses on standardising the development of clinical guidelines and quality standards.

Using NICE to support evidence-based practice – a social work case study
Rachel Scourfield is a consultant social worker at Neath Port Talbot County Borough Council. Rachel outlines the work she’s been doing to embed NICE's evidence-based recommendations.

Planning for the future – a resource impact case study
Mohammed Asghar, prescribing governance lead at Frimley Health and Care Integrated Care System, and Carolyn Craven, prescribing support pharmacist at Cheshire and Merseyside Health and Care Partnership, discuss our resource planning tools.

Co-existing mental illness and substance misuse – a health inequalities case study
Jane Bethea, consultant in public health at Nottinghamshire Healthcare NHS Foundation Trust, outlines the work of Nottinghamshire’s substance misuse and mental health pathway development group.
Health technology assessment in Latin America
In this case study, we explore NICE International's collaboration with several Latin American countries. This work focuses on challenging aspects of health technology assessment.

Engaging to improve: outcomes of the young adult diabetes clinic restructure
Learn how The Royal Liverpool and Broadgreen University Hospitals NHS Trust used our guidance and quality standards to improve outcomes in young adult diabetes care.

Accelerated Access Collaborative – using our evidence standards framework in the Artificial Intelligence in Health and Care Award
Dr Emma Hughes explains how the Accelerated Access Collaborative within NHS England has incorporated the NICE evidence standards framework for digital health technologies into their Artificial Intelligence in Health and Care Award.

Health Technology Wales - using our evidence standards framework in topic selection
Dr David Jarrom explains how Health Technology Wales has incorporated our evidence standards framework for digital health technologies into its topic selection process for technology appraisals.

Developing and adapting clinical guidelines in India
We explore NICE International's collaboration with the Indian Health Outcomes, Public Health and Economics Research Centre. This project focuses on providing support for guideline development in the field of eye health.
Implementing health technology assessment in Egypt
Learn about NICE International’s work with the Egyptian Authority for Unified Procurement, Medical Supply and the Management of Medical Technology. We’ve been working collaboratively to implement health technology assessment in the country.

Developing health technology assessment in Denmark
Discover how NICE International has worked with Danish stakeholders to further improve health technology assessment process and methods.

Contextualising NICE guidelines in Cyprus
We reveal how NICE International has been working with Cyprus' Health Insurance Organisation to develop clinical guidelines and quality performance indicators.

Reveal LINQ - a digital health developer’s journey through NICE
We speak to Medtronic’s Mark Chapman and Lisa Jones who reflect on Reveal LINQ’s™ journey through our Diagnostics Assessment Programme.

Zio XT - From NICE digital health pilot to AI Award winner
iRhythm Technologies was the first company to take their artificial intelligence (AI) technology – Zio XT – through the NICE digital health technologies guidance development pilot. We speak to the team involved to learn about iRhythm’s journey through the NICE guidance process and to hear about what’s next for this innovative technology as part of the AI in Health and Care Award.
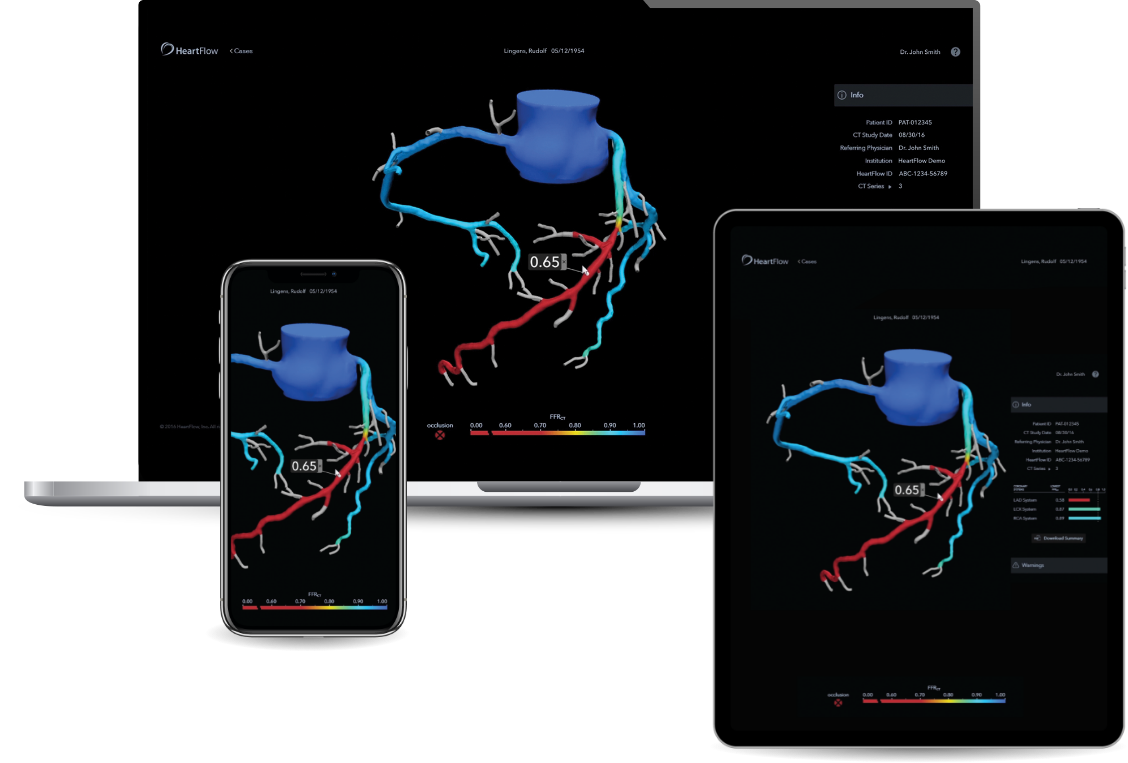
HeartFlow’s journey from NICE guidance to MedTech Funding Mandate
We chat with Gina McDonald Main and Campbell Rogers from HeartFlow. They reflect on their product’s journey through our Medical Technologies Evaluation Programme and provide advice for other medical technology companies considering submitting their product for NICE evaluation.